
Universal plasma project recognised with award
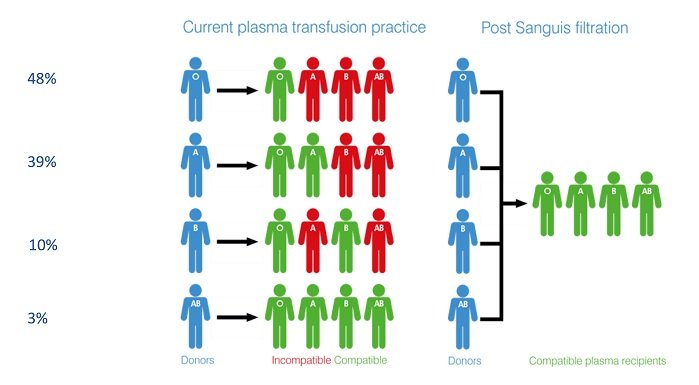
The successful production of universal plasma, the aim of the Sanguis project, would enable hospitals to keep a single stock of blood.
13th September 2018
Innovation in Textiles
|
Leeds
Leading a consortium of experts in their respective fields, the Nonwovens Innovation & Research Institute (NIRI) has reached successful proof-of-concept in developing a new nonwoven filter technology – through the Sanguis project – to remove antibodies from donated blood and so produce universal plasma.
Health services must hold sufficient stocks of different blood group plasma to ensure an adequate supply to meet clinical demands and to ensure patient safety. In addition to logistical challenges, this current situation presents a potential risk to patients, should transfused blood components be incorrectly matched.
The successful production of universal plasma, the aim of the Sanguis project, would enable hospitals to keep a single stock of blood which, addressing the issues noted above, would reduce the risk of transfusion delays as well as avoiding wastage and cutting administrative costs – savings estimated at £30 million, in the UK alone. Potentially, emergency services could carry universal plasma for use in the golden hour, the 60-minute period during trauma patient treatment when medical intervention has the greatest chance of saving life.
Blood processing involves the separation of blood into plasma and red blood cell components using centrifugation. The plasma is subsequently filtered through a multi-layered nonwoven mattress to remove Leukocytes (white blood cells) present in blood, which cause side effects for transfusion recipients. Blood groups are derived from specific antigens present on the red blood cell membrane surface.
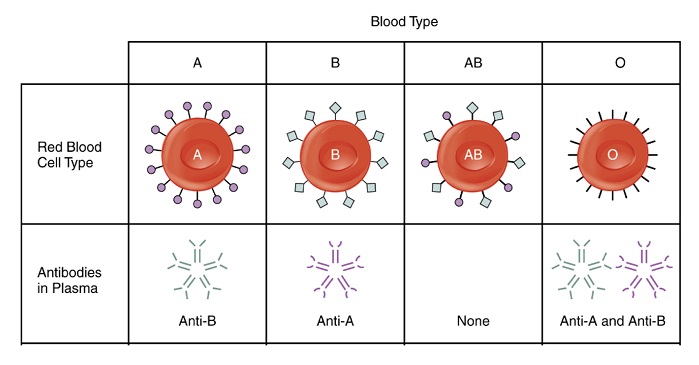
The body produces antibodies for the antigens not present on its red blood cells, and the antibodies are dissolved in the blood plasma. Antibodies (immunoglobulins) bind specifically to foreign bodies causing opsonisation, increasing the probability that the foreign body will undergo phagocystosis and be removed. Removal of these antibodies bypasses the immune response, allowing safe transfusion.
The Sanguis filter uses an artificial antigen that mimics the structure of the naturally occurring antigens found in red blood cells, incorporating NIRI’s patented linker technology and nonwoven expertise. The process for producing universal plasma involves functionalisation of conventional meltblown filter media with the artificial antigen, welding into hard plastic casing and, finally, sterilisation.
Meltblown nonwovens are relatively inexpensive and existing specifications can be used without the need for expensive and lengthy modification to production protocol. A thermoplastic fibre-forming polymer is extruded via a linear die containing hundreds of small orifices. Convergent streams of hot air rapidly attenuate the extruded polymer streams to form extremely fine diameter fibres which are collected on a conveyor, where an autogenously-bonded meltblown nonwoven is formed. The fibres in the meltblown web are bonded by a combination of entanglement and cohesive sticking.
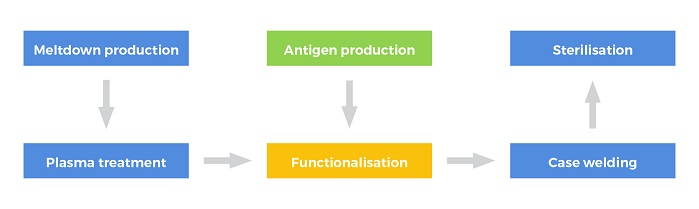
During the functionalisation process, the filament surface in the nonwoven was plasma treated, introducing carboxylic acid groups onto the fibre surfaces, providing a grafting point for other species, i.e. the antigen. Quantification of functionality has been determined and the process has resulted in a loading level in excess of that required for antigen immobilisation. Attachment of the antigen to the activated meltblown was conducted using aqueous coupling chemistry to immobilise the antigen to the PBT substrate allowing recovery of any unreacted antigen from the residual solution.
Measurement was conducted using methodology developed in collaboration with NHS Blood and Transplant UK (NHSBT), a Sanguis consortium partner. Based on agglutination, fresh plasma was tested both before and after filtration to assess the effectiveness of antibody removal. Two different antigens were challenged with two immunoglobulin antibodies, and the results showed high efficiency removal of the target antibodies, with residual antibody levels being consistent with delivery of universal plasma (≤1:16).
To ensure products are safe for medical use, sterilisation is required in all medical applications to deactivate all living biological agents. While current blood filters are sterilised using either beta or steam processes, both methods can reduce the filtration efficiency of meltblown nonwovens.
Assessing both methods, beta sterilisation was found to be more effective for the Sanguis filter, causing less of a reduction in efficacy compared to steam sterilisation. Further safety testing was carried out by NHSBT. Safety and biological testing assessed the effect of filtration on seven parameters in the blood and confirmed no negative impacts on the quality of the blood plasma following the filtration process. The filters did not adversely affect the plasma, as proteins were not removed, and clotting pathways were not activated.
The project involved several partners and draws on the expertise and unique knowledge of each. Chemistry specialist Carbosynth, leading blood filter manufacturer Macopharma (UK) and NHSBT have worked with NIRI to bring about the success that Sanguis has demonstrated.
“The Sanguis nonwoven filter medium and associated filtration technology has been demonstrated for removal of target antibodies from donated human blood plasma sufficient to deliver universal plasma – the goal of the project,” commented Dr Matthew Tipper, Business Director at NIRI.
“This has huge potential to benefit health services – increasing efficiency, reducing costs and improving patient safety, and with the potential to help save lives. We are looking, too, at additional applications for the technology as the fundamental approach developed, tested and proven here could be modified to provide a platform technology for selective filtration for other liquids and, in the first instance, we are considering applications for fuel filtration, food and beverage and for the wider pharmaceutical industry.”
Business intelligence for the fibre, textiles and apparel industries: technologies, innovations, markets, investments, trade policy, sourcing, strategy...
Find out more